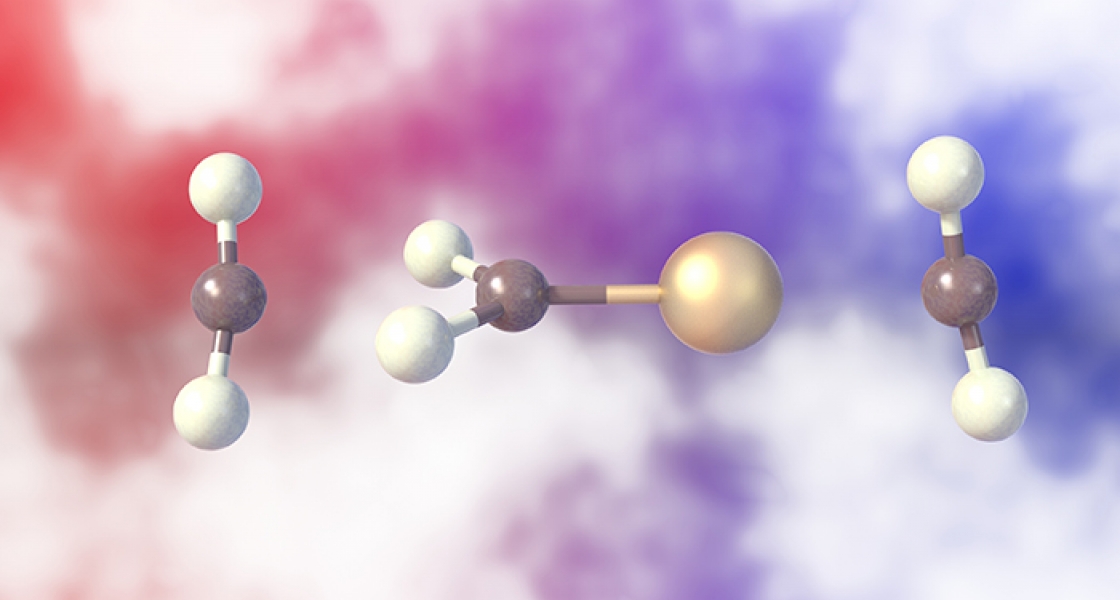
Graduate student Ben Knurr and Fellow Mathias Weber have added new insight into a catalytic reaction based on a single gold atom with an extra electron that transfers this electron into carbon dioxide molecules (CO2). This reaction could be an important first step future industrial processes converting waste CO2 back into chemical fuels. As such, it could play a key role in a future carbon-neutral fuel cycle.
What the Weber group did was use vibrational spectroscopy to probe the effect of solvent CO2 molecules as they came in contact with a gold-CO2 complex (AuCO2-). This complex is the first step in a catalytic reaction of gold and CO2 that adds back chemical energy to a CO2 molecule.
Before the researchers even did the experiment, they knew that if solvent molecules of CO2 attach themselves to the CO2 end of the AuCO2- complex, they would enhance the chances of the CO2 acquiring an extra electron, freeing itself from the gold atom, and completing the first step of the conversion reaction. The newly formed and highly reactive (i.e., activated) CO2- ions could then be used in a series of additional steps to make recycled liquid fuels.
Knurr and Weber found that the first eight solvent molecules of CO2 preferentially attached themselves around the CO2 end of the AuCO2- complex. As the number of solvent molecules increased from 1 to 8, the activation of the complex-bound CO2 also intensified, enabling it to grab more and more of the needed electron. The activation reaction was most favored when all eight solvent molecules were in place around the complex.
But, adding even one more solvent molecule around the complex diminished the chances of activation. The reason was that there was no longer any room around the complex-bound CO2 for more solvent. Additional solvent molecules ended up around the gold atom, where they pulled the extra electron nearer the gold atom, diminishing the chances for activation of the bound CO2.
This work clearly shows how solvation can both enhance and diminish the effects of a catalyst like gold. It promises to inform the design and engineering of industrial systems aimed at creating carbon-neutral fuel cycles. — Julie Phillips